Abstract
Acute lymphoblastic leukemia (ALL) is the most common childhood hematological malignancy and is associated with high morbidity and mortality in adults. In the last years, extensive efforts have been made to define the genetics of ALL in order to identify lesions contributing to leukemogenesis and treatment failure. The translocation t(1;19), coding for the oncogenic fusion protein E2A (TCF3)-PBX1, determines a block at the B-cell precursor differentiation stage, and is associated with newly described pre-B cell receptor (pre-BCR) phenotype. Relapse in patients with E2A-PBX1 occurs very frequently in central nervous system (CNS). Therefore, there is a medical need for the identification of active substances in CNS for the treatment of E2A-PBX1+/pre-BCR+ ALL. Recent studies have indicated that the SRC-family kinases inhibitor dasatinib could be effective both in vitro and in vivo in E2A-PBX1+/pre-BCR+ ALL.
Aim: In this project, we aimed to identify mechanism of sensitivity and resistance to dasatinib in E2A-PBX1+/pre-BCR+ and to establish combination of active therapies in CNS. We employed genetic and pharmacologic approaches in vitro and secondary transplantation assays with murine E2A-PBX1+/pre-BCR+ leukemia cells in vivo.
Methods: Using an unbiased shRNA library screen approach, we identified BTK as key protein involved in dasatinib sensitivity and validated it in shRNA knock-down- and sgRNAs CRISPR/Cas9 knock-out-based competition assays. We assessed the effect of combined therapy in vitro and in vivo with dasatinib and BTK inhibitors (BTKi), which are described to penetrate in CNS. We performed using H&E and GFP immunostainings, phospho-specific flow cytometry (phospho-flow), western blotting, bulk RNA sequencing and mass spectrometry.
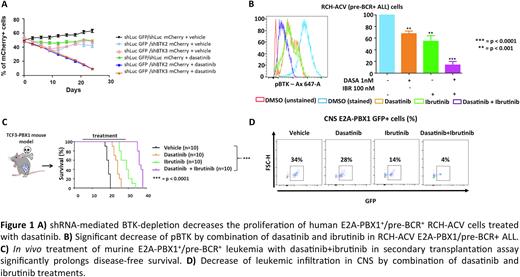
Results: BTK-depleted cells by shRNAs and sgRNAs showed decreased proliferation of dasatinib-treated E2A-PBX1+ cells compared with control-transduced cells. E2A-PBX1+/pre-BCR+ human and murine cells were more sensitive to dasatinib in combination with BTKi therapy (ibrutinib, acalabrutinib or zanubrutinib) than E2A-PBX1-/pre-BCR- ALLs as shown by cell proliferation and phospho-flow assays. The combination of dasatinib with BTKi significantly increased the disease-free survival of mice in secondary transplantation assays and reduced the leukemic infiltration, especially in CNS, but also in bone marrow, lymph nodes, spleen and liver as demonstrated by flow cytometry and immunostainings.
Conclusions: Genetic depletion and pharmacological inhibition of BTK increase dasatinib sensitivity in human and mouse E2A-PBX1+/pre-BCR+ ALL and the combination of dasatinib and BTKi is very effective especially reducing CNS-infiltration of ALL cells in vivo.
Disclosures
Luebbert:AbbVie: Honoraria; Astex: Honoraria; Janssen: Research Funding; Otsuka: Consultancy; Syros: Consultancy; Cheplapharm: Other: study drug. Duque Afonso:Roche: Consultancy, Honoraria; Amgen: Honoraria; AstraZeneca: Honoraria; Riemser: Honoraria; Lilly: Honoraria; IPSEN: Honoraria; SOBI: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal